What is soda Ash?
soda ash is a chemical compound known as an inorganic combination of sodium and carbonate. The chemical formula of soda ash is (Na2CO3). This substance is available in solid, crystalline, powdery and odorless form and has the property of absorbing moisture. Soda ash is produced industrially in almost the whole world and it is not possible to produce it at home. In its solid state, its soda appears as a white powder. And when sodium carbonate dissolves in water, carbonic acid and sodium hydroxide are formed. In this article, we are going to answer the important question, what is soda ash? Stay with us to answer completely.
soda ash is used in many industries. One of the important uses of soda ash is in the glass industry. Also, it is used in the soap industry, paper production, pH adjustment, production of cleaners and detergents, and in the polymer and resin industries. In general, soda ash is a chemical compound with diverse applications that is used as a material in many industries and chemical processes. Important is used.
History
soda ash has been known since ancient times. In ancient times, the ashes of plants and wood were burned in a fire, and then the resulting ashes were mixed with water and evaporated to obtain sodium carbonate. This process was known as “Kahwashi”.
The first industrial production of sodium carbonate from plant ash started in Europe. This process was widely used in the glass and soap making industries.
In 1791, French chemist Nicolas Labans prepared sodium carbonate in its present form. He extracted sodium carbonate from sea ash in a new way and named the result “sodium carbonate”. In the late 19th century, industrial methods were developed to produce sodium carbonate. Among these methods, we can mention the Solway process using ammonia, limestone and salt, which is still in use today. Sodium carbonate as a material with various applications, including glass production, soap making, paper making, cleaners and chemical industries. , is still available and used.
Physical and chemical characteristics of sodium carbonate
The melting temperature of sodium carbonate is 851 degrees Celsius. This product decomposes at higher temperatures. The solubility of sodium carbonate in water at 20 degrees Celsius is equal to 215 grams per liter.
Physical characteristics

Form: Its soda is available as crystalline or white powder. These crystals are usually hexagonal or elliptical in shape.
Appearance: In crystalline form, soda is shiny and transparent. In powder form, the appearance is opaque and similar to white powder.
Solubility: Sodium carbonate is soluble in water. When dissolved in water, it forms sodium ions (Na+) and carbonate ions (CO3–).
Melting point: Sodium carbonate has a melting point of about 851 degrees Celsius. At this temperature, sodium carbonate changes from a solid state to a liquid state.
Density: The density of sodium carbonate is about 2.54 grams per cubic centimeter.
Solubility in other substances: sodium carbonate is soluble in some other substances, such as alcohol and glycerin.
Solution pH: When sodium carbonate dissolves in water, an alkaline solution with a pH of about 11 to 12 is formed.
The physical characteristics of sodium carbonate are related to visual properties, solubility, melting temperature, density and solution pH. These characteristics can be variable, especially in different temperature and pressure conditions.
Chemical characteristics

Chemical formula: Na2CO3
Molecular weight: approximately 105.99 g/mol
Appearance: white, crystalline, odorless powder
Melting point: 851 degrees Celsius
Boiling point: Decomposes at temperatures above 1600 degrees Celsius
Solubility: soluble in water, alcohol, glycerin.
pH of 10% aqueous solution: about 11-12
Flash point: non-flammable
Chemical properties: soda ash is a strong alkali and has the ability to neutralize acids.
Common uses: production of glass, soap, detergent products, petrochemical industry, water treatment, pH adjustment, sulfur treatment, etc.
Types of soda ash
For industrial applications, two types of sodium carbonate are used, which are as follows:
- Heavy soda ash
- Light soda ash
The difference between heavy soda ash and light sodium carbonate is only in density, particle size and their application, and they are not chemically different from each other.
Heavy sodium carbonate has a mass density of about 1000 kg/m3 and particles with a size of 300 to 500 microns.
This type of carbonate is often used in glass factories. Coarse granule particles like this type cause the absence of dust and impurities and reduce the possibility of particles spreading during transportation.
The density of light soda ash is about 500 kg/m3 and the size of light sodium carbonate particles is about 100 microns.
Applications of soda ash

Sodium carbonate (Na2CO3) is used in various industries and applications. Some of its main
uses are:
- food industry
- water refinery
- pH adjustment
- Aluminum industry
- Textile industry
- Oil and gas industry
- Cleaning and household
- Polymer production
- Desulfurization
- Refinement of vegetable oils
- Toothpaste production
- Taxidermy
- electrolyse
- Glasswork
- Payroll production
- Papermaking
food industry
Sodium carbonate is used in the food industry in the form of summary in the following applications
- Adjusting the pH of food products.
- Adjusting the level of acidity in pickles and increasing their shelf life.
- Use in baking products such as bread, cakes and biscuits.
- Use as a seasoning powder in carbonated drinks.
- Use as a diluent in sauces, preserves and jams.

These uses may vary depending on the type of product and the relevant health standards and laws
water purification
soda ash is used in water purification as follows:
- Adjust the pH of purified water.
- Water softener to reduce water hardness.
- Descaling and preventing the formation of insoluble sediments.
- Use in salt water purification to make salt water usable and drinkable.
In any case, the use of soda ash should be done in accordance with the regulations and sanitary standards of the region and the water treatment process.
pH adjustment
soda ash is used in pH adjustment in some food products and related industries. Some of the products in which sodium carbonate is used as a pH regulator are:
- Bread and baked goods: soda ash may be used as a pH adjuster in the production of bread, cakes, biscuits and other baked goods. This material facilitates to improve the consistency and final structure of the product.
- Pickles: In the production of pickles such as pickles, pickled cucumbers, olives and other pickled products, soda ash is used to adjust the level of pickling and increase shelf life.
- Carbonated mineral water: soda ash is used as a seasoning powder in some carbonated drinks such as carbonated mineral water. This substance creates bubbles by releasing carbon dioxide and thus carbonated water is produced
- Sauces and Jams: soda ash may be used as a diluent in the production of sauces, preserves and jams. This material improves the consistency and dilution of the product.
Aluminum industry
In the aluminum industry, sodium carbonate is used as a chemical. Some of the applications of sodium carbonate in the aluminum industry are:

- Purification and cleaning of aluminum: soda ash is used as a cleaning agent. By adding soda ash to water or chemical solutions used in aluminum production processes, it is possible to remove various pollutants such as oil, sediments and suspended particles.
- Aluminum foil production: In the aluminum foil production process, soda ash is used as one of the chemicals. This substance is used in some stages of the aluminum foil production process to adjust pH, cool and control chemical processes.
- Removal of iron oxide: in some stages of aluminum production and processing, iron oxide may be present as a pollutant. Sodium carbonate can be used as a chemical to remove iron oxide and help purify and purify aluminum.
Textile industry
soda ash is used in various sectors in the textile industry. Its applications include fiber pretreatment, dyeing, fiber polishing, and rinsing pH adjustment. The use of sodium carbonate is used to adjust the pH in the rinsing and washing stages of cotton fibers, as a color remover in the dyeing process of fibers, as a finishing agent to stabilize the color on the fibers, and to adjust the pH of fiber rinsing. The use of soda ash in the textile industry should be done in accordance with the relevant standards and guidelines.
Oil and gas industry

Sodium carbonate is used in the oil and gas industry as a material with multiple applications. Its applications include adjusting the pH of solutions, preventing the formation of sediments, regulating the mass of the reservoir and using it in drilling processes. The use of sodium carbonate in this industry requires compliance with relevant standards and guidelines.
.
Cleaning and household
soda ash in home cleaning can be used to clean and whiten surfaces, clean kitchen utensils, clean floors and carpets, and clean iron surfaces. When using sodium carbonate, instructions and safety tips should be followed.
Polymer production
soda ash is used as a sodium source and catalyst in the production of polymers. Its main applications include the production of polyvinyl chloride (PVC), polyethylene terephthalate (PET), polyacrylonitrile (PAN) and polyurethane. By using soda ash in these processes, the characteristics and properties of the final polymers can be adjusted and the production quality can be improved.
Desulfurization

soda ash is used in desulfurization. This substance can be used as a neutralizing gas in industrial processes. Also, it is used in fuels to remove sulfur and prevent the production of polluting gases related to sulfur. Sodium carbonate is also used in water and wastewater treatment to remove sulfur. In the chemical industry, soda ash is also used to remove sulfur in production processes. In any case, in using sodium carbonate for desulfurization, it is necessary to pay attention to the relevant instructions and safety tips and observe the appropriate amounts.
Refinement of vegetable oils
- Adjusts the pH of the oil.
- Removes the color and impurities in the oil.
- Separates oil and water.
- Removes the salts in the oil.
When using soda ash in the purification of vegetable oils, follow the instructions and safety tips and pay attention to the appropriate amounts.
Toothpaste production
soda ash is used in the production of toothpaste as a material with the following applications:

- Adjusting mouth pH and neutralizing acids.
- Cleaning and disinfection of teeth and gums.
- Preventing the formation of plaque and the deposition of color on the teeth.
- Adding the right flavor to the toothpaste.
taxidermy
soda ash in taxidermy to:
- Act antibacterial and antifungal.
- Adjust the pH of the environment.
- Preserves and softens the animal’s skin.
- Prevent cracks and deformation of the skin.
In any case, when using soda ash in taxidermy, follow the instructions and safety tips and use the appropriate amounts.
electrolyse
soda ash is used as an electrolyte in electrolysis. Below is a brief explanation of the use of sodium carbonate in electrolysis:
- Electrolyte: soda ash is used as an electrolyte in the electrolysis process. In electrolysis, an electric current is passed through the electrolyte medium so that the ions move separately towards the electrodes.
- Ionization: sodium carbonate forms sodium (Na+) and carbonate (CO3^2-) ions in the presence of water. In electrolysis, these ions move individually to the electrodes and participate in chemical reactions.
- Decomposition and combination of ions: In the electrolysis process, sodium ions move to the cathode and receive electrons, while carbonate ions move to the anode and lose
- This process breaks down sodium carbonate into sodium (Na) and carbon dioxide (CO2).
Glasswork
Sodium carbonate is one of the main and key materials in the glass industry. Below is a brief explanation about the use of sodium carbonate in glass making:

- Alloying agent: sodium carbonate is used as an alloying agent in the preparation of glass. By adding sodium carbonate to the mixture of glass raw materials, the melting temperature of glass decreases and the process of glass purification is accelerated.
- pH regulation and stabilization: sodium carbonate is used as a pH regulator in the glass production process. This material can reduce the effect of acids in the raw materials and adjust the pH of the environment, which can contribute to the stability and better quality of the glass.
- Increasing the thermal stability: by adding sodium carbonate to the glass, the thermal stability of the glass increases. This property can make the glass more resistant to thermal changes and prevent cracks and undesirable deformation.
- Improving glass melting ability: sodium carbonate is used as an auxiliary material in the glass melting process. This material can reduce the melting temperature of glass and improve the melting process.
- Glass color control: sodium carbonate can play a role in glass color control. By adjusting the amount of sodium carbonate, the amount of glass color can be changed and different colors of glass can be produced.
In general, sodium carbonate is used in the glass industry to control and improve the properties of glass and helps to produce glass with the desired quality and properties.
Brick production
soda ash is used as an additive or filler in the brick manufacturing industry. Below is a brief explanation about the use of sodium carbonate in brick production:
Dough stabilization: sodium carbonate is used as a filler in brick production dough. By adding sodium carbonate to the dough, the rheological properties (flow and plasticity control) of the dough are improved, and the casting and shaping capabilities are improved.
Reduction of cracking: sodium carbonate can reduce corrosion cracks in brick. Adding sodium carbonate to the dough increases thermal stability and resistance to thermal changes in the baking process and reduces cracks.
Reducing water absorption: sodium carbonate can be used as a filler that helps reduce water absorption in concrete. This feature can increase resistance to moisture, reduce expansion and contraction due to water absorption, and improve construction properties of brick.
Oxidation control: soda ash can prevent brick oxidation. By adding sodium carbonate to the dough, the oxidation process of the brick during baking is reduced and the color and final quality of the brick are improved.
paper making
Sodium carbonate is used in the paper industry as follows:
- Adjust the pH of the paper.
- Increasing the whiteness and brightness of the paper.
- Increasing the mechanical strength of paper.
- Control of water absorption by paper.
- Improving the printability of paper.
These are the important things that sodium carbonate facilitates to improve the properties and quality of paper.
Production of sodium carbonate
Sodium carbonate is a chemical compound that is widely used in various industries, including chemical industry, glass industry, food industry and paper industry. The production of sodium carbonate is carried out by two main methods, i.e. the Solovey process and the Lime process.
Solvay Process:
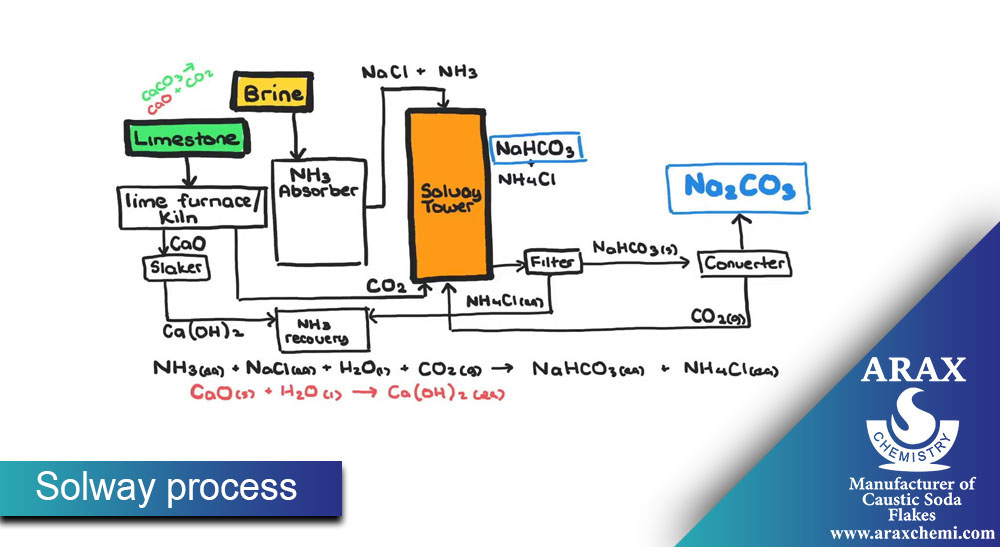
In the Solovey process, soda ash synthesis is done from raw materials such as sodium chloride, sodium carbonate, and ammonia. The main steps of this process are:
Preparation of placebo (Brine): First, sodium chloride (table salt) is dissolved in water and placebo (solvent containing salt) is prepared.
Preparation of Solum (Ammonia-Soda Ash Solution): Solum is prepared by combining soda ash, ammonia and placebo.
Solum decomposition: Solum is decomposed in equipment such as decomposition towers to decompose sodium carbonate.
Extraction of sodium carbonate: sodium carbonate is extracted from solem as a final product. After extraction, sodium carbonate is available as a solid, liquid or solvent.
Lime Process:
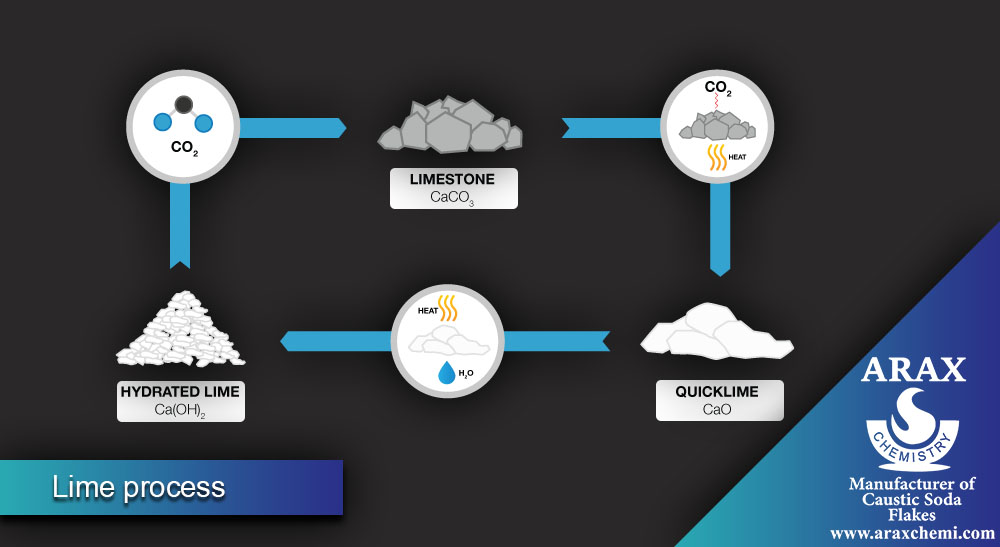
In the lime process, soda ash is also produced, but using different raw materials. The main steps of this process are:
Cooling and purifying the placebo: The prepared placebo (sodium chloride in water) is cooled and purified from impurities.
Addition of calcium carbonate: Calcium carbonate is added to the placebo, which forms the semi-product of soda ash as a calcium carbonate precipitate.
Separation of soda ash: The precipitate formed is separated from the solution and then converted into pure soda ash using methods such as purification and drying.
Innovations of soda ash
Recent efforts have been made to optimize soda ash production processes using greener and more sustainable methods and technologies. This research has been carried out in order to reduce energy consumption, reduce environmental effects and improve resource efficiency. Researches in the field of improving soda ash purification methods in order to remove pollution and impurities have been investigated. This research includes the use of advanced filtration, absorption and extraction processes of contaminated materials and the use of new catalysts. New uses of soda ash: Recent research has been done in the field of new uses of soda ash. For example, the use of sodium carbonate in the production of recyclable and reusable batteries, use in catalytic processes, use in nano technologies, etc. are among the things that have been investigated.
Improving the quality and properties of soda ash: Research has been conducted in the field of improving the quality and properties of soda ash. Such as improving purity and degradability, optimizing physical and chemical properties, improving resistance to moisture and heat, etc.
It is important to know that these researches and innovations are based on the fields studied and developed, and more detailed information about them may be available in scientific sources and related articles.
last word
soda ash is an important chemical used in many industries, including glass, soap, detergent, paper, food, and petrochemical industries. In vegetable oil refining processes, soda ash plays an important role as a substance used in oil separation, acid neutralization, pH adjustment and purification. In desulfurization processes, soda ash is used as a key ingredient to remove sulfur from fossil fuels, such as crude oil and natural gas, and improve fuel quality. Today, the lime method is used more due to its higher efficiency and lower energy consumption. soda ash, as an important and multifunctional chemical, has a significant effect in various industries, and points such as applications, production processes, and its effects on final products are discussed in the article.
Therefore ,soda ash as a multi-purpose material with wide applications in various industries and its positive effect on processes and products is an important issue.
ARAX CHEMISTRY company is a great manufacturer of Caustic Soda Flakes, that can offer its High-quality products.