Inorganic Compounds vs organic Compounds
Wherever we look around, we can see both inorganic and organic compounds. When we go to a supermarket, we see different items; some are organic while others are inorganic. What is the difference between organic and inorganic compounds?
Well, the chemical difference is not the one you hear on the news which distinguishes “organic” vegetables from “non-organic” ones. Guess what, both are made up of organic and inorganic compounds.
Let’s say that the “agriculture industry” definition is not the same as the chemical definition. In chemistry, there is a major difference, which is well defined.
Telling the difference between organic and inorganic compounds is one of the main things you need to make clear while learning chemistry. If you are interested, learn more about thermodynamics and kinetics, another two of the three most important concepts in chemistry.
In this article, we will explain it in detail, so in the end, you will be able to differentiate both types of chemicals without any difficulty. We will try to solve all your doubts about this eternal chemistry question!
In the early days, scientists separated organic and inorganic compounds on the fact that the first group was considered as a result of the activity of living beings, whereas the second group belonged to processes unrelated to any way of life. Now there are much clearer definitions.
In the early days, scientists separated organic and inorganic compounds on the fact that the first group was considered as a result of the activity of living beings, whereas the second group belonged to processes unrelated to any way of life. Now there are much clearer definitions.
Organic Compounds
Inorganic Compounds vs organic Compounds
An easy, layman-friendly definition for organic compounds is that those are the ones that are derived from living things such as plants and animals are known as organic compounds like sugars, lipids, proteins, nucleic acids, etc.
More strictly speaking, we consider a compound to be organic if it is made of carbon atoms that participate in covalent bonds. Generally, (but not always), organic compounds also present covalent C–H bonds.
Inorganic Compounds
An easy definition for an outsider, is that those compounds which are obtained from non-living things or mineral sources are known as inorganic compounds like NaCl (table salt) and NaHCO3, (baking soda), etc.
Defining inorganic compounds is pretty easy after having defined organic compounds. As a rule, every chemical that does not fall into the category of “organic”, is considered an inorganic compound.
The Modern Definitions
Organic Compounds
The compounds which contain carbon atoms as the main constituent, which are bonded together through covalent bonds, are called organic compounds. Most organic compounds also contain hydrogen. Other common elements present in organic compounds are oxygen, nitrogen, Sulphur, halogens, and phosphorous. But those are not the only ones.
In most cases, all atoms of the different elements are held together through covalent bonds. Some exceptions would be, for example, organic carboxylates, or ammonium salts. But you could argue that those are “inorganic salts of organic compounds”.
Some compounds that might sound “non-organic as hell” such as polymers (a fancy name for plastics) are actually long-chained organic compounds. An example is polystyrene. Its backbone is basically all covalent C–C and C–H bonds.
Bear in mind that “organic compound” does not imply “biochemical compound”. On the other hand, the backbone of biochemistry is mostly organic compounds (although metals are extremely important in biological systems such as iron in hemoglobin).
Inorganic compounds
Inorganic Compounds vs organic Compounds
Take every organic compound out. You are left with inorganic compounds. If it doesn’t fall into the definition of organic, it is inorganic.
In general, the compounds which do not have C–C or C–H covalent bonds are called inorganic compounds.
There are many compounds that only have covalent bonds, they have carbon atoms, but are not organic compounds. Examples of this type of inorganic compounds include carbon monoxide, carbon dioxide, inorganic carbonates, carbides, etc. Notably, allotropes of carbon such as graphite, graphene, or diamond, contain only carbon atoms but are considered inorganic compounds.
As you can see, sometimes the definition is not so well established. In fact, I couldn’t really find a clear definition for both provided by IUPAC. This illustrates the fact that defining the line between inorganic and organic chemicals.
Some interesting examples of this middle ground are organometallic compounds. These are made up of an organic component, generally bound to an inorganic component through a carbon-metal bond. These are really fun and are one of the most widely explored research topics in modern chemistry!
Examples of Organic Compounds or Molecules
Molecules associated with living organisms are organic. These include nucleic acids, fats, sugars, proteins, enzymes, and hydrocarbon fuels. All organic molecules contain carbon, nearly all contain hydrogen, and may also contain oxygen.
DNA
table sugar or sucrose, C12H22O11
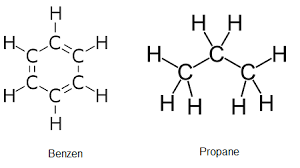
benzene, C6H6
methane, CH4
ethanol or grain alcohol, C2H6O
Examples of Inorganic Compounds
Inorganics include salts, metals, substances made from single elements, and any other compounds that don’t contain carbon bonded to hydrogen. Some inorganic molecules do, in fact, contain carbon.
table salt or sodium chloride, NaCl, Calcium Chloride
carbon dioxide, CO2
diamond (pure carbon)
sulfur
sodium hydroxide(NaOH)
ARAX CHEMISTRY is a great manufacturer of caustic soda Flakes, Aluminum Sulfate, and Copper Sulfate which offers its High-quality products.
Source: chemistry hall