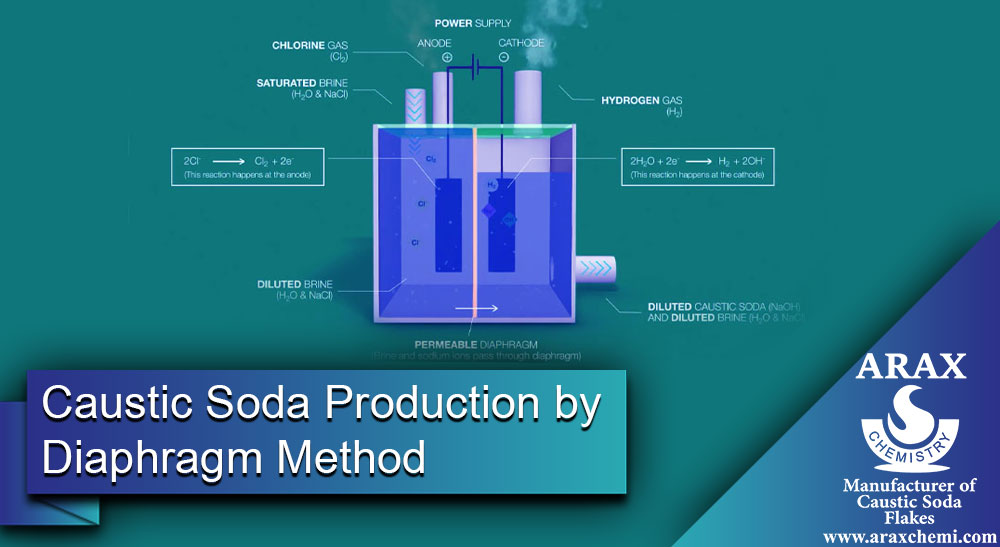
Caustic Soda Production by Diaphragm Method
Caustic soda, which is also known as sodium hydroxide, can be produced in different ways. In this article, we are going to explain how caustic soda is produced by using the diaphragm method. The diaphragm method involves the use of a diaphragm (a thin separating layer) between two volumes of liquid, which leads to the separation of ions and the formation of caustic soda. In this method, sodium chloride salt (NaCl) is used as a raw material. First of all, we need to get acquainted with the concept of diaphragm and how to make it.
What is a diaphragm?
In general, a diaphragm is an isolation structure that is divided into two different parts to prevent interference between different components of a system, but allows the passage of some substances or ions between these two parts. Diaphragms act as an ionic buffer in the electrolysis process that separates the electrolyte flow into two separate parts, the cathodic and the anodic. The main function of the diaphragm is to separate the ions to prevent their combination and interference, yet allowing the ions to pass through. These ions can be positively charged (cations) or negatively charged (anions) and in the process of electrolysis, the diaphragm acts as a separation between these two types of ions. Among the most important diaphragms, polymer diaphragms and cellulose diaphragms can be mentioned.
- Polymer diaphragms: they are usually made of polymer materials such as Nafion. These types of diaphragms have semi-permeable properties, meaning they allow ions to pass through and prevent larger particles such as electrolytes and solids from passing through.
- Cellulose diaphragms: They are also made of materials such as carboxymethyl cellulose (CMC). These diaphragms have the property of absorbing water and with the help of this property, they perform the separation of ions in the electrolysis process.
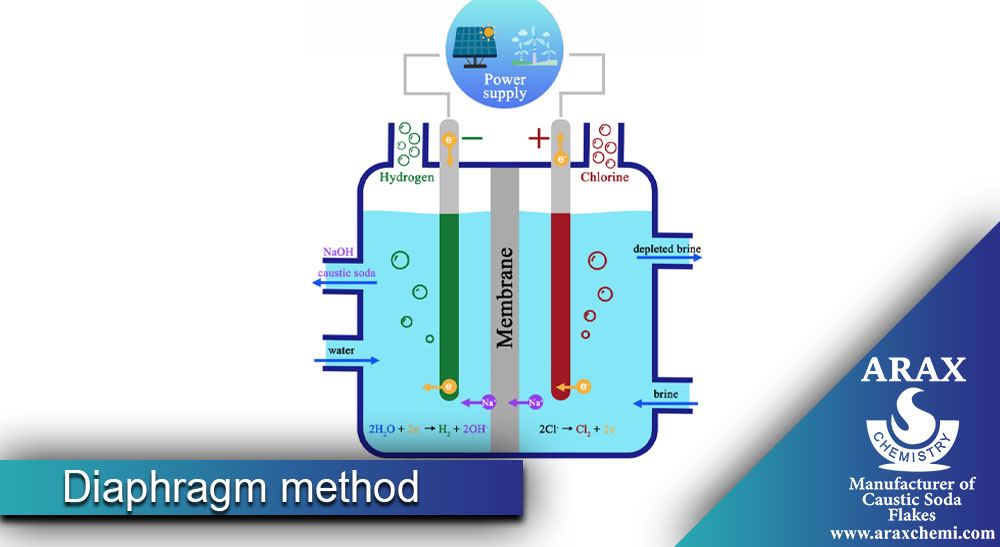
The difference between polymer and cellulose diaphragms
Polymer and cellulose diaphragms have different properties. The main differences between these two types of diaphragms will be explained.
- Materials:
Polymer diaphragms are made of polymer materials such as polyvinyl chloride (PVC) or polymethyl methacrylate (PMMA). These polymers are usually obtained from petroleum sources and have suitable mechanical and chemical properties for diaphragm process.
Cellulosic diaphragms are made from materials such as carboxymethyl cellulose (CMC). Cellulose is usually obtained from plant sources such as pulp, cotton and wood. Cellulose diaphragms are suitable for using in the diaphragm process due to the structure of cellulose and its properties such as water absorption.
- Chemical stability:
Polymer diaphragms usually have higher chemical stability than cellulosic diaphragms. They are resistant to stronger solvents such as alcohols, aldehydes and acids. This feature is very important to prevent diaphragm destruction in the electrolysis process.
In contrast, cellulosic diaphragms may be more vulnerable to solvents and harsher chemical conditions. They may need to be replaced in alkaline and hot environments over time.
- Ionic permeability:
Polymer diaphragms usually have lower ion permeability than cellulosic diaphragms. This means a reduction in the passage of ions through the diaphragm and a better separation of ions in the electrolysis process.
Cellulose diaphragms have more ion permeability due to their specific cellulosic structure.
- Cost and production capability:
Polymer materials are widely produced in the market, which can lead to the development and use of polymer diaphragms more easily.
In contrast, cellulosic diaphragms may cause a higher production cost. The production of cellulose and its processing into cellulosic diaphragms require more complex processes that may put limits in the development and use of these types of diaphragms.
On the other hand, both types of diaphragms are used as ion separators in the process of electrolysis and caustic soda production, but depending on the properties and materials used, they may have different applications. In some electrolysis cells, polymer diaphragms such as Nafion are used. These diaphragms are semi-permeable, meaning they allow ions to pass through and prevent larger particles such as electrolyte from passing through. Choosing the right material for the diaphragm depends on the specific process needs and operating conditions. Below we examine some other materials used to make the diaphragm:
Other diaphragm construction materials
- Polymers: Polymers are among the common materials used to make diaphragms. Some of the polymers used include polyvinyl chloride (PVC), polyethylene (PE), polypropylene (PP), polytetrafluoroethylene (PTFE) and polysulfone (PSU).
- Refractories: Refractory materials are also used as diaphragms in some processes. Examples of refractories include silica, alumina, zirconia, and carbon.
- Polymers containing ions: Some polymers that have functional groups forming ions can also be used as diaphragms in ion separation processes. Examples of these polymers include polysulfonate, polyethylene glycol (PEG) and polystyrene sulfonate (PSS).
- Composite membranes: Composite membranes include a combination of several materials and can be used as a diaphragm. These membranes usually consist of polymers reinforced with materials such as silica, alumina, or carbon.
The most suitable material for making a diaphragm depends on the process conditions and specific needs. Factors such as chemical stability, mechanical resistance, permeability, proper size of pores and porosity, ability to separate ions, and ability to recycle and reuse may affect the selection of the right material. For selecting the right material for a diaphragm, it is usually necessary to consider the operating conditions used, the required properties (such as chemical resistance, mechanical resistance, thermal stability, and separability), and the costs associated with the material.
Steps of caustic soda production by diaphragm method
1- :Preparing the diaphragm
First, you need to prepare a suitable diaphragm to separate the ions. The diaphragm can be made of different materials such as polymers, refractories and semi-permeable materials.
2- :Preparation of saline solution
At this point, you need to prepare a saline solution. To produce caustic soda, sodium chloride salt is used. Dissolve salt in water and make a saline solution.

3- Division of liquid volume:
Divide the volume of saline solution into two parts. Put one part in one container and put the other part in another container.
4- Placing the diaphragm:
Place the diaphragm between two containers containing a volume of liquid. The diaphragm must allow the passage of sodium ions (Na+) from the positive side to the negative side (where caustic soda is formed) and prevent the passage of chlorine ions (Cl-).
5- Application of electric current:
By applying an electric current in the two containers, the sodium ions move from the positive side to the negative side and are separated by the diaphragm along the way. In this process, chlorine ions also move to the positive side and combine with hydroxide ions (OH-) formed on the negative side, and caustic soda is produced.
6- Separation of caustic soda:
After applying the electric current and forming the caustic soda, you can separate the caustic soda. To do this, separate the two containers and collect the caustic soda from the negative side of the diaphragm.
7- Drying and packing:
Heat the caustic soda to dry and then pack it properly.
Advantages and disadvantages of producing caustic soda by using diaphragm metod
Advantages
- High efficiency: the diaphragm method is highly effective in the production of caustic soda. By using this method, it is possible to produce chlorine and sodium hydroxide simultaneously and with high efficiency.
- Electrolyte separation: Using a diaphragm as a separating layer between electrolytes in the electrolysis process allows the separation of chlorine and sodium hydroxide. This precise separation can help to obtain pure and high quality products.
- Less energy consumption: The diaphragm method generally requires less energy consumption than other caustic soda production methods. This advantage is achieved by separating the electrolytes and the electric current through the diaphragm.

Disadvantages
- High investment cost: Setting up and operating diaphragm systems for the production of caustic soda requires a high investment cost. The complex structure of the diaphragm and the need for special equipment can increase the initial costs.
- Operational problems: Diaphragm systems may face operational problems. For example, creating hydraulic pressure to maintain the pressure inside the electrolysis cells requires precise control of the electric current and ensuring the stability of the diaphragm between the electrolytes.
- Electrolyte flow density: using a diaphragm may cause a limitation in electrolyte flow density. This limitation can lead to a limitation in the production of chlorine and sodium hydroxide and have an impact on the scalability of the process.
The advantages and disadvantages of the diaphragm method in the production of caustic soda depend on the specific conditions of each process and specific needs. To decide whether to use the diaphragm method, you need to compare its advantages and disadvantages with other caustic soda production methods and consider your specific conditions.
Other methods of producing caustic soda
In addition to the diaphragm method, the production of caustic soda can be done using other methods. Two other famous methods for producing caustic soda are:
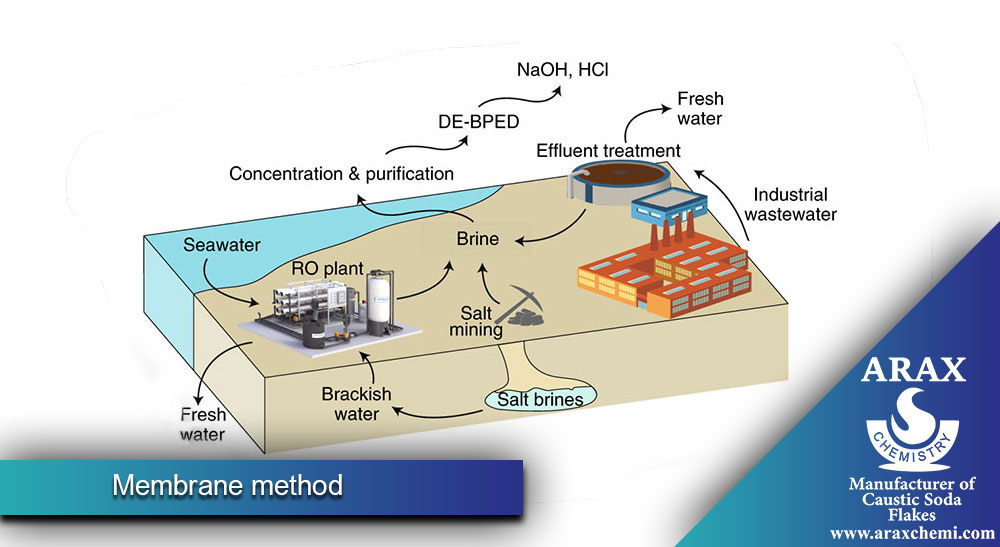
- :Membrane Process
In this method, ion-selective membranes are used to separate the electric current. In this method, chlorine and hydroxide ions are separated through the membrane. The advantage of this method is that it does not require a diaphragm and therefore the problems related to the diaphragm such as failure and the need for maintenance and replacement are less. Also, this method generally consumes less energy. However, the cost of manufacturing and maintaining membranes can be high.
- :Direct Extraction Process
In this method, a separating agent is used to separate chlorine and hydroxide ions in the electrolysis flow. Typically, sulfur is also used as a separating agent. This method can have lower investment and maintenance costs than the diaphragm method. However, this method requires the use of new chemicals and more complex processes
Each of these methods has its advantages and disadvantages, and depending on the specific conditions and industrial needs, one method may be more suitable than the other. Choosing the right method for producing caustic soda should be done considering the factors such as costs, efficiency, sustainability and environmental needs.
last word
Producing caustic soda using the diaphragm method has advantages and disadvantages. Its advantages include high efficiency, separation of electrolytes, and lower energy consumption. But its disadvantages include high investment cost and operational problems. In addition to the diaphragm method, there are other methods to produce caustic soda. Membrane method using ionic membranes and direct extraction method using separating materials can be named. Choosing the right method for caustic soda production should be done according to specific conditions and industrial needs. Factors such as costs, efficiency, sustainability and environmental needs must be considered.
In general, choosing the right method for producing caustic soda should be done considering technical, economic and environmental factors. Each method has its advantages and disadvantages and should be selected according to the specific needs and operating conditions.