What is the degree of purity?
The degree of purity or degree of purity of a material shows the degree of purity and pollution of that material. This parameter indicates the presence of other compounds (impurities) in the desired material. The degree of purity is the most important indicator used to evaluate the quality of a chemical substance.
The degree of purity is expressed as a percentage or fraction and shows that a percentage of the desired substance is formed. For example, if a substance has a purity of 99%, it means that 99% of the weight of the substance is pure, and the remaining 1% consists of impurities and other compounds.
Purity is very important for various chemicals, from organic and inorganic to pharmaceutical and electronic materials. In some industries, the need for high-purity materials is critical, as impurities and other compounds may have a detrimental effect on the required performance.
To measure the degree of purity, various methods such as spectroscopy, chromatography and chemical analyzes are used. These methods allow us to examine materials and determine their purity.
What is caustic soda?
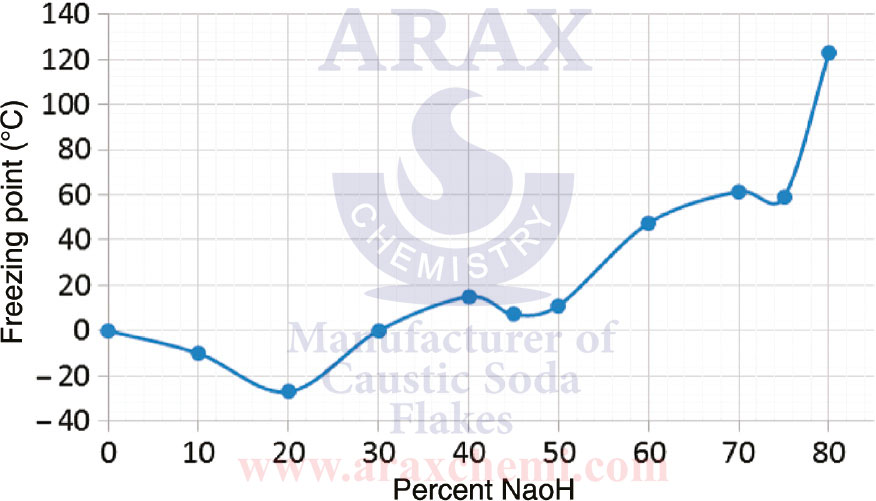
Caustic soda or sodium hydroxide (NaOH) is a strong chemical compound with alkaline properties. This compound is used in solid form as white and crystalline powder and in aqueous solution as liquid powder. Caustic soda is one of the strongest and most common corrosives.
Caustic soda is used as an important chemical in various industries. Some of its main uses are:
- Soap industry: Caustic soda has long been used in soap production as an essential alkali.
- Chemical industries: This compound is used in the production of various chemicals such as polymers, paints, resins and as a catalyst in chemical processes.
- Water purification: Caustic soda is used as a strong alkali in water purification processes to neutralize the acids in the water and adjust the pH.
- Paper industry: In paper production, caustic soda is used as an alkaline substance to absorb dyes and acids in the paper bleaching process.
- Textile industry: Caustic soda is used in the process of dyeing and cleaning textile fabrics.
It is important to know that caustic soda is a strong alkaline substance and should be used with caution and safety guidelines, because it can cause risks such as skin and eye irritation, burns and damage to sensitive tissues.
Purity of caustic soda
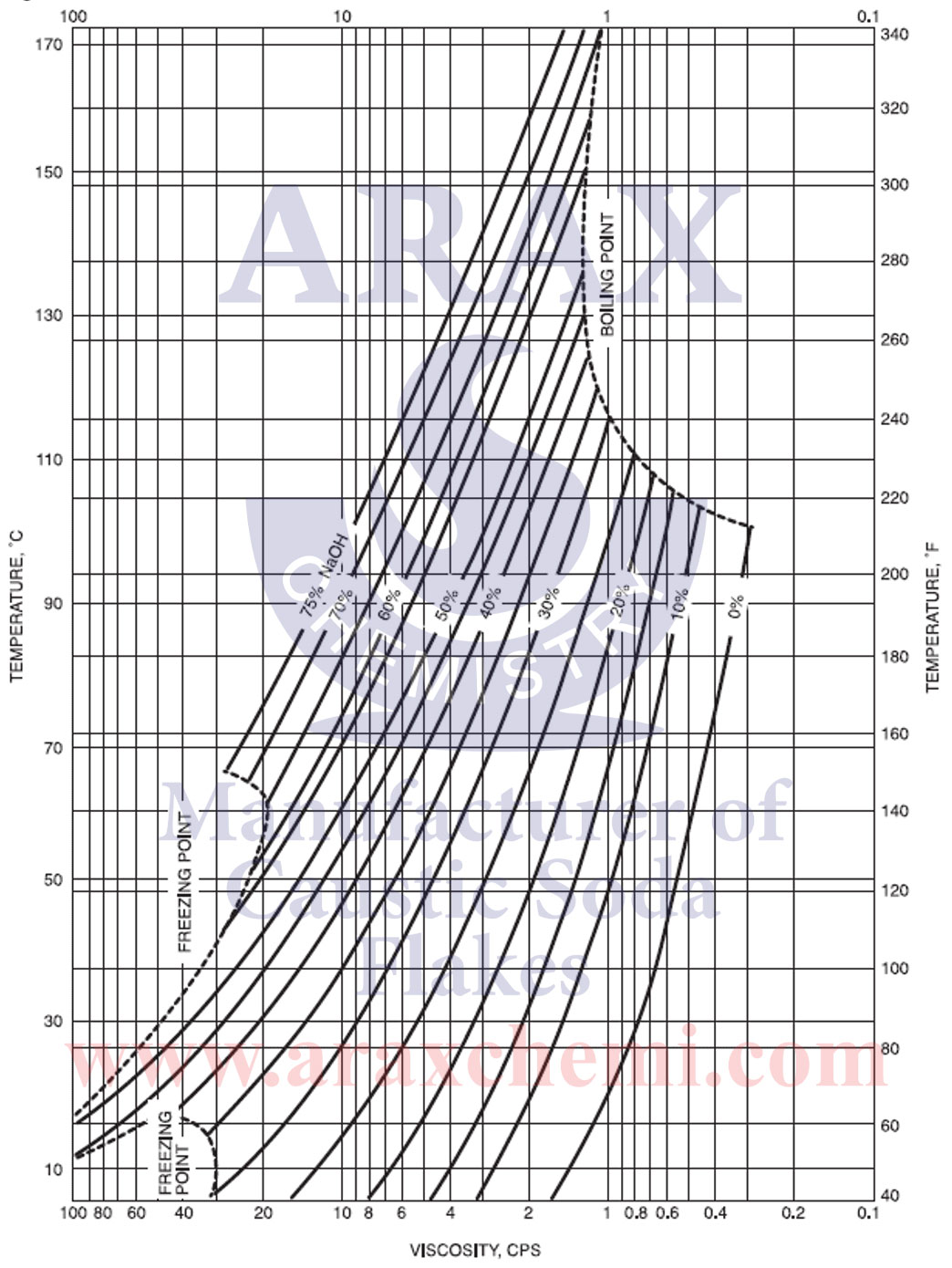
The degree of purity of caustic soda or sodium hydroxide (NaOH) indicates the degree of purity and pollution of this chemical compound. The degree of purity is usually expressed as a percentage and indicates that a percentage of the weight is caustic soda and the rest is impurities and other compounds. The degree of purity of caustic soda can be different for different applications. For example, when buying caustic soda in the market, its degree of purity is generally specified as a percentage. For industrial uses and chemical industries, even a higher degree of purity is required.
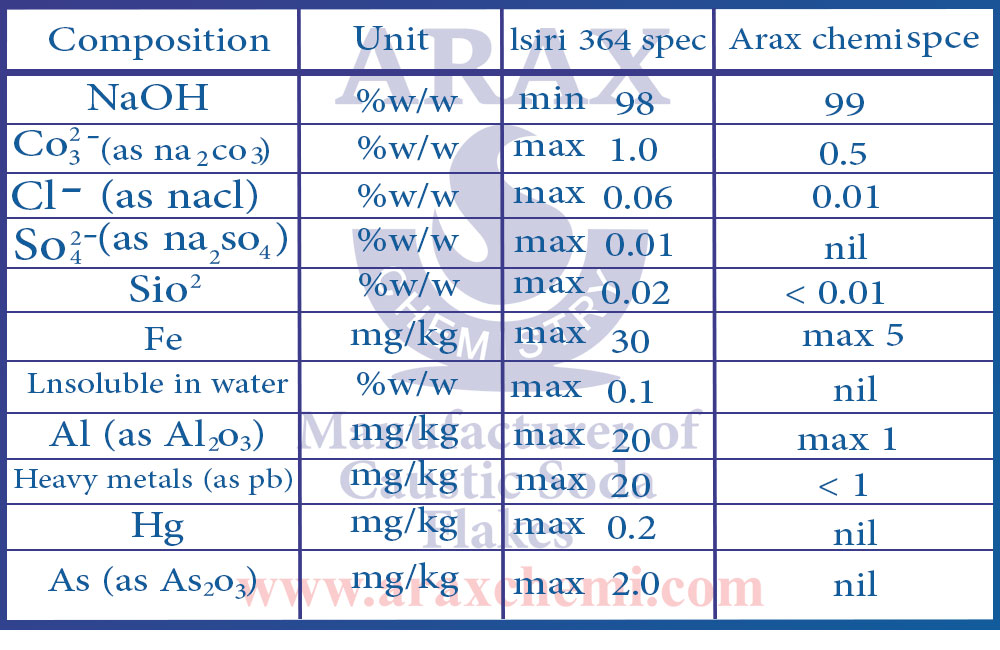
The purity of caustic soda can range from 98%, 99%, 99.5% and even higher to as high as 99.9% or even higher for some specific applications. To measure the purity of caustic soda and determine the amount of impurities, various methods such as chemical analyzes and qualitative and quantitative tests are used. Also, the standards and technical specifications related to caustic soda determine their own degree of purity, based on which the purity of caustic soda can be checked.
It is important to know that a higher degree of purity indicates the quality and purity of caustic soda, so for some applications that require higher purity, caustic soda with a higher degree of purity is chosen.
Tools and equipment necessary to measure the degree of purity
1- Sampler: PTFE membrane filter, 37 mm with a pore size of 1 micron (Millipore, Fluoropore or similar types),
With cellulose backing pad, in cassette filter holder.
2- Individual sampling pump with a flow rate of 1-4 L/min, along with flexible connecting pipes
3- PH meter with PH electrode and recorder
4- titration container; 150 to 200 ml beaker or balloon,
along with a cover that has a valve for the PH electrode as well as N2 inlet and outlet
5- Magnetic stirrer
6- glass rod, with a diameter of 5 mm and a length of 10 cm,
To keep the filter below the liquid level in the titration container
7- 5 and 10 ml pipettes
8- 100 ml and 1 liter balloons
9- 50 ml burette with a graduation of 0.1 ml
10- Pliers.
Calibration and quality control
Calibration and quality control are two related concepts in the field of measuring and ensuring the accuracy and correctness of the results. Next, I will explain each one:

calibration
Calibration means adjusting and adapting a measuring instrument to specific standards. The main purpose of calibration is to ensure the accuracy and correctness of measurement results. During calibration, a measuring instrument (such as a thermometer, weight, pH meter, meter, etc.) is compared using a valid and accurate standard. With this, the accuracy of the measuring instrument is checked and, if needed, its settings and digital settings are adjusted to provide more accuracy and precision to the measurement results. Calibration should be performed periodically and regularly to ensure that the measuring instrument is consistently accurate.
quality control
Quality control refers to the set of activities and methods that guarantee the quality of the product or service at each stage of the production process or performing an activity. The main purpose of quality control is to ensure that the product or service meets the standards and needs of customers. To achieve this goal, quality control processes include steps such as sampling, testing and measuring, comparing results with established criteria, modifying processes if necessary, and recording relevant data. Using quality control, it is ensured that products and services are consistently delivered with acceptable quality and that customer needs are met.
Both calibration and quality control are used as part of quality management systems to improve the accuracy, precision, and quality of products and services, and to improve customer satisfaction of the products they provide.
Normality of hydrochloric acid stock solution
The normality of the hydrochloric acid (HCl) stock solution indicates the concentration of hydrochloric acid in the stock solution. Reference stock (stock) is used as a standard solution that has a certain concentration of a chemical compound and is used for calibration of instruments and measurements in laboratories. Normality is defined as the number of moles in one liter of solution. Therefore, the normality of the hydrochloric acid stock solution indicates the number of moles of hydrochloric acid per liter of solution. For example, a stock solution of hydrochloric acid with a normality of 1 can contain 1 mole of hydrochloric acid (HCl) per liter of solution.
Normality is also used to determine the concentration of an unknown solution. According to determining the normality of the hydrochloric acid stock solution and using titration methods, the required volume of the stock solution can be used to neutralize or determine the concentration of another solution. Caution is usually required when using stock solutions of hydrochloric acid because hydrochloric acid is concentrated and strong and can cause serious damage to skin, eyes, and laboratory equipment. Therefore, when using it, observe safety and relevant safety regulations.
Purity measurement
degree of purity
1- Reverse the titration of the excess hydrochloric acid in the main sample, control and spiked samples with a standardized (homogeneous) caustic soda solution. At the same time, purge with nitrogen.
2- At the same time as the titration, look at the pH meter. Determine the end point (ml of normal 0.01 caustic soda used).
The interlopers
Carbon dioxide in the air may react with alkalis on the filter to form carbonates, but will not interfere with the titration. Carbonates can cause positive interference. Acidic particles can neutralize the sample and have negative interference.
Calculations
Calculate the concentration of caustic soda in the air using the following equation:
C= ((V_(NaOH-b)- V_(NaOH-S) ).N × 40 × 〖10〗^3)/V
In this regard:
C = caustic soda concentration in mg/m3
VNaOH-b = the volume of caustic soda in the control sample titration in milliliters
VNaOH-s = volume of caustic soda in the titration of the original sample in milliliters
N = normality of caustic soda titration solution
40 = molecular weight of sodium bicarbonate
V = volume of air sampled in liters
Conclusion
In conclusion, the article talked about the concept and importance of degree of purity. The degree of purity is a very important indicator in science and industry that shows how pure and unadulterated a substance is. This concept is used in various fields such as chemistry, physics, pharmaceutical and electronics industries. To determine the degree of purity of a substance, various methods are used, including chemical analysis, analysis, and physical tests. These methods allow us to accurately determine the degree of purity of a material and identify its strengths and weaknesses.
The degree of purity can have a great impact on the performance and efficiency of products and industrial processes. Improving the degree of purity of raw materials and finished products can improve the quality, accuracy and repeatability of tests and processes. This is very important in achieving higher standards and customer satisfaction. Finally, the degree of purity of a material can be considered as an important parameter in the design, production and quality control of products and industrial processes. Continuous review and improvement of the degree of purity of materials and products leads to the progress and development of various industries and helps to improve the efficiency and competitiveness of companies.v

