Soda ash is an inorganic chemical compound composed of sodium and carbonate, with the chemical formula (Na2CO3). This substance is available in solid, crystalline, powdered, and odorless forms and has moisture-absorbing properties. soda ash is produced industrially worldwide, and home production is not feasible. In its solid form, soda ash appears as a white powder. When soda ash dissolves in water, it forms carbonic acid and sodium hydroxide.
Like caustic soda, soda ash has numerous applications in various industries. One of its significant uses is in the glass manufacturing industry. Additionally, it is utilized in soap production, paper manufacturing, pH regulation, the production of cleaners and detergents, and in the polymer and resin industries. Overall, soda ash is a chemical compound with diverse applications, serving as an important substance in many industries and chemical processes.
Brief History of Soda Ash
Sodium carbonate has been known since ancient times. In ancient periods, people burned the ashes of plants and wood, then mixed the resulting ashes with water and evaporated it to obtain soda ash. This process was known as “kahavashi.” The first industrial production of soda ash from plant ashes began in Europe, and this method was widely used in the glass and soap industries.
In 1791, French chemist Nicolas Leblanc prepared soda ash in its modern form. He developed a new method to extract sodium carbonate from sea ashes and named the result “soda ash.” By the late 19th century, industrial methods for producing sodium carbonate were developed, including the Solvay process, which uses ammonia, limestone, and salt, and is still in use today. Sodium carbonate remains a versatile compound with applications in glass manufacturing, soap production, paper making, cleaners, and the chemical industry.
Physical and chemical characteristics of sodium carbonate
-

Physical soda ash
How to Identify Soda Ash (Physical and Chemical Properties)
The melting point of sodium carbonate is 851 degrees Celsius. This product decomposes at higher temperatures. The solubility of sodium carbonate in water at 20 degrees Celsius is 215 grams per liter.
Physical Properties of Soda Ash
- Appearance: White, odorless powder or crystalline solid.
- Solubility: Highly soluble in water, with a solubility of 215 g/L at 20°C.
- Melting Point: 851°C (1564°F).
- Density: Approximately 2.533 g/cm³ in solid form.
- State at Room Temperature: Solid.
These properties help in the identification and application of soda ash in various industrial processes.
- Form: Its soda is available as crystalline or white powder. These crystals are usually hexagonal or elliptical in shape.
- Appearance: In crystalline form, soda is shiny and transparent. In powder form, the appearance is opaque and similar to white powder.
- Solubility: Sodium carbonate is soluble in water. When dissolved in water, it forms sodium ions (Na+) and carbonate ions (CO3–).
- Melting point: Sodium carbonate has a melting point of about 851 degrees Celsius. At this temperature, sodium carbonate changes from a solid state to a liquid state.
- Density: The density of sodium carbonate is about 2.54 grams per cubic centimeter.
- Solubility in other substances: sodium carbonate is soluble in some other substances, such as alcohol and glycerin.
- Solution pH: When sodium carbonate dissolves in water, an alkaline solution with a pH of about 11 to 12 is formed.
The physical characteristics of sodium carbonate are related to visual properties, solubility, melting temperature, density and solution pH. These characteristics can be variable, especially in different temperature and pressure conditions.
Chemical characteristics
-

Chemical soda ash
- Chemical Formula: Na2CO3.
- Reaction with Water: Dissolves in water to form carbonic acid (H2CO3) and sodium hydroxide (NaOH).
- pH Level: Aqueous solutions of soda ash are alkaline, typically having a pH above 10.
- Decomposition: At elevated temperatures, sodium carbonate decomposes into sodium oxide (Na2O) and carbon dioxide (CO2).
Types of soda ash
For industrial applications, two types of sodium carbonate are used, which are as follows:
- Heavy soda ash
- Light soda ash
Differences in Applications of Heavy and Light Soda Ash
The differences between heavy and light soda ash lie primarily in their density, particle size, and applications, while chemically, they are the same.
Heavy Soda Ash
- Bulk Density: Approximately 1000 kg/m³.
- Particle Size: Particles range from 300 to 500 microns.
- Applications:
- Predominantly used in glass manufacturing.
- The larger granule size minimizes dust and impurities, reducing the likelihood of particle dispersion during transport.
Light Soda Ash
- Bulk Density: Approximately 500 kg/m³.
- Particle Size: Particles are about 100 microns in size.
- Applications:
- Used in various chemical processes, including soap production, detergents, and water treatment.
- The finer particle size enhances solubility and reactivity in aqueous solutions.
Overall, while both types of soda ash serve important roles in industry, their physical properties dictate their specific applications and handling characteristics.
uses of soda ash in industry

Soda ash (sodium carbonate) has a wide range of applications across various industries, uses of soda ash including:
- food industry
- water refinery
- pH adjustment
- Aluminum industry
- Textile industry
- Oil and gas industry
- Cleaning and household
- Polymer production
- Desulfurization
- Refinement of vegetable oils
- Toothpaste production
- Taxidermy
- electrolyse
- Glasswork
- Payroll production
- Papermaking
food industry
Sodium carbonate is used in the food industry in the form of summary in the following applications:
- Adjusting the pH of food products.
- Adjusting the level of acidity in pickles and increasing their shelf life.
- Use in baking products such as bread, cakes and biscuits.
- Use as a seasoning powder in carbonated drinks.
- Use as a diluent in sauces, preserves and jams.

water purification
soda ash is used in water purification as follows:
- Adjust the pH of purified water.
- Water softener to reduce water hardness.
- Descaling and preventing the formation of insoluble sediments.
- Use in salt water purification to make salt water usable and drinkable.
In any case, the use of soda ash should be done in accordance with the regulations and sanitary standards of the region and the water treatment process.
pH adjustment
soda ash is used in pH adjustment in some food products and related industries. Some of the products in which sodium carbonate is used as a pH regulator are:
- Bread and baked goods: soda ash may be used as a pH adjuster in the production of bread, cakes, biscuits and other baked goods. This material facilitates to improve the consistency and final structure of the product.
- Pickles: In the production of pickles such as pickles, pickled cucumbers, olives and other pickled products, soda ash is used to adjust the level of pickling and increase shelf life.
- Carbonated mineral water: soda ash is used as a seasoning powder in some carbonated drinks such as carbonated mineral water. This substance creates bubbles by releasing carbon dioxide and thus carbonated water is produced
- Sauces and Jams: soda ash may be used as a diluent in the production of sauces, preserves and jams. This material improves the consistency and dilution of the product.
Aluminum industry
In the aluminum industry, sodium carbonate is used as a chemical. Some of the applications of sodium carbonate in the aluminum industry are:

- Purification and cleaning of aluminum: soda ash is used as a cleaning agent. By adding soda ash to water or chemical solutions used in aluminum production processes, it is possible to remove various pollutants such as oil, sediments and suspended particles.
- Aluminum foil production: In the aluminum foil production process, soda ash is used as one of the chemicals. This substance is used in some stages of the aluminum foil production process to adjust pH, cool and control chemical processes.
- Removal of iron oxide: in some stages of aluminum production and processing, iron oxide may be present as a pollutant. Sodium carbonate can be used as a chemical to remove iron oxide and help purify and purify aluminum.
Textile industry
soda ash is used in various sectors in the textile industry. Its applications include fiber pretreatment, dyeing, fiber polishing, and rinsing pH adjustment. The use of sodium carbonate is used to adjust the pH in the rinsing and washing stages of cotton fibers, as a color remover in the dyeing process of fibers, as a finishing agent to stabilize the color on the fibers, and to adjust the pH of fiber rinsing. The use of soda ash in the textile industry should be done in accordance with the relevant standards and guidelines.
Oil and gas industry

Sodium carbonate is used in the oil and gas industry as a material with multiple applications. Its applications include adjusting the pH of solutions, preventing the formation of sediments, regulating the mass of the reservoir and using it in drilling processes. The use of sodium carbonate in this industry requires compliance with relevant standards and guidelines.
Cleaning and household
soda ash in home cleaning can be used to clean and whiten surfaces, clean kitchen utensils, clean floors and carpets, and clean iron surfaces. When using sodium carbonate, instructions and safety tips should be followed.
Polymer production
soda ash is used as a sodium source and catalyst in the production of polymers. Its main applications include the production of polyvinyl chloride (PVC), polyethylene terephthalate (PET), polyacrylonitrile (PAN) and polyurethane. By using soda ash in these processes, the characteristics and properties of the final polymers can be adjusted and the production quality can be improved.
Desulfurization

soda ash is used in desulfurization. This substance can be used as a neutralizing gas in industrial processes. Also, it is used in fuels to remove sulfur and prevent the production of polluting gases related to sulfur. Sodium carbonate is also used in water and wastewater treatment to remove sulfur. In the chemical industry, soda ash is also used to remove sulfur in production processes. In any case, in using sodium carbonate for desulfurization, it is necessary to pay attention to the relevant instructions and safety tips and observe the appropriate amounts.
Refinement of vegetable oils
- Adjusts the pH of the oil.
- Removes the color and impurities in the oil.
- Separates oil and water.
- Removes the salts in the oil.
When using soda ash in the purification of vegetable oils, follow the instructions and safety tips and pay attention to the appropriate amounts.
Toothpaste production
soda ash is used in the production of toothpaste as a material with the following applications:

- Adjusting mouth pH and neutralizing acids.
- Cleaning and disinfection of teeth and gums.
- Preventing the formation of plaque and the deposition of color on the teeth.
- Adding the right flavor to the toothpaste.
taxidermy
soda ash in taxidermy to:
- Act antibacterial and antifungal.
- Adjust the pH of the environment.
- Preserves and softens the animal’s skin.
- Prevent cracks and deformation of the skin.
In any case, when using soda ash in taxidermy, follow the instructions and safety tips and use the appropriate amounts.
electrolyse
soda ash is used as an electrolyte in electrolysis. Below is a brief explanation of the use of sodium carbonate in electrolysis:
- Electrolyte: soda ash is used as an electrolyte in the electrolysis process. In electrolysis, an electric current is passed through the electrolyte medium so that the ions move separately towards the electrodes.
- Ionization: sodium carbonate forms sodium (Na+) and carbonate (CO3^2-) ions in the presence of water. In electrolysis, these ions move individually to the electrodes and participate in chemical reactions.
- Decomposition and combination of ions: In the electrolysis process, sodium ions move to the cathode and receive electrons, while carbonate ions move to the anode and lose
- This process breaks down sodium carbonate into sodium (Na) and carbon dioxide (CO2).
Glasswork
Sodium carbonate is one of the main and key materials in the glass industry. Below is a brief explanation about the use of sodium carbonate in glass making:

- Alloying agent: sodium carbonate is used as an alloying agent in the preparation of glass. By adding sodium carbonate to the mixture of glass raw materials, the melting temperature of glass decreases and the process of glass purification is accelerated.
- pH regulation and stabilization: sodium carbonate is used as a pH regulator in the glass production process. This material can reduce the effect of acids in the raw materials and adjust the pH of the environment, which can contribute to the stability and better quality of the glass.
- Increasing the thermal stability: by adding sodium carbonate to the glass, the thermal stability of the glass increases. This property can make the glass more resistant to thermal changes and prevent cracks and undesirable deformation.
- Improving glass melting ability: sodium carbonate is used as an auxiliary material in the glass melting process. This material can reduce the melting temperature of glass and improve the melting process.
- Glass color control: sodium carbonate can play a role in glass color control. By adjusting the amount of sodium carbonate, the amount of glass color can be changed and different colors of glass can be produced.
In general, sodium carbonate is used in the glass industry to control and improve the properties of glass and helps to produce glass with the desired quality and properties.
Brick production
soda ash is used as an additive or filler in the brick manufacturing industry. Below is a brief explanation about the use of sodium carbonate in brick production:
- Dough stabilization: sodium carbonate is used as a filler in brick production dough. By adding sodium carbonate to the dough, the rheological properties (flow and plasticity control) of the dough are improved, and the casting and shaping capabilities are improved.
- Reduction of cracking: sodium carbonate can reduce corrosion cracks in brick. Adding sodium carbonate to the dough increases thermal stability and resistance to thermal changes in the baking process and reduces cracks.
- Reducing water absorption: sodium carbonate can be used as a filler that helps reduce water absorption in concrete. This feature can increase resistance to moisture, reduce expansion and contraction due to water absorption, and improve construction properties of brick.
- Oxidation control: soda ash can prevent brick oxidation. By adding sodium carbonate to the dough, the oxidation process of the brick during baking is reduced and the color and final quality of the brick are improved.
paper making
Sodium carbonate is used in the paper industry as follows:
- Adjust the pH of the paper.
- Increasing the whiteness and brightness of the paper.
- Increasing the mechanical strength of paper.
- Control of water absorption by paper.
- Improving the printability of paper.
These are the important things that sodium carbonate facilitates to improve the properties and quality of paper.
Production of sodium carbonate
Sodium carbonate is a chemical compound that is widely used in various industries, including chemical industry, glass industry, food industry and paper industry. The production of sodium carbonate is carried out by two main methods, i.e. the Solovey process and the Lime process.
Solvay Process:
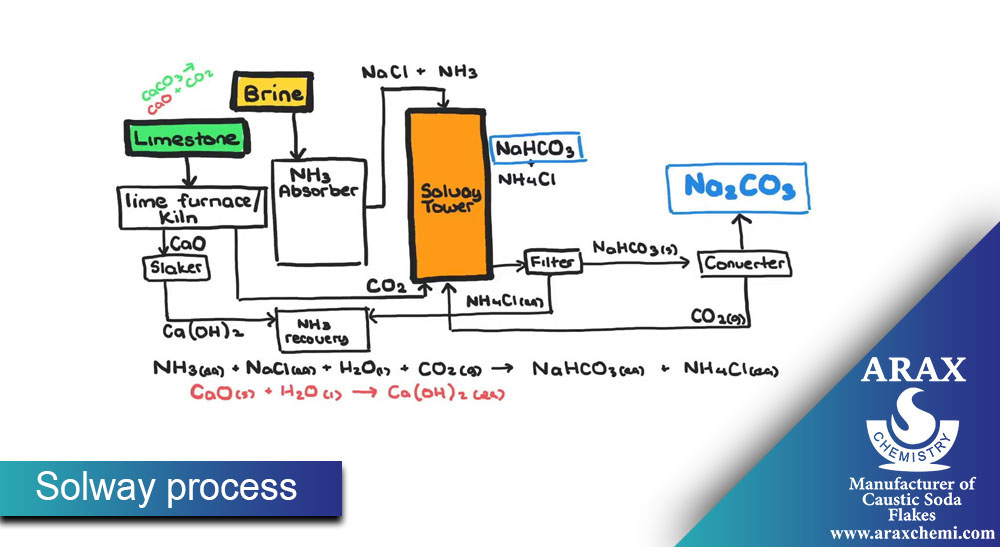
In the Solovey process, soda ash synthesis is done from raw materials such as sodium chloride, sodium carbonate, and ammonia. The main steps of this process are:
Preparation of placebo (Brine): First, sodium chloride (table salt) is dissolved in water and placebo (solvent containing salt) is prepared.
Preparation of Solum (Ammonia-Soda Ash Solution): Solum is prepared by combining Sodium carbonate, ammonia and placebo.
Solum decomposition: Solum is decomposed in equipment such as decomposition towers to decompose sodium carbonate.
Extraction of sodium carbonate: sodium carbonate is extracted from solem as a final product. After extraction, sodium carbonate is available as a solid, liquid or solvent.
Lime Process
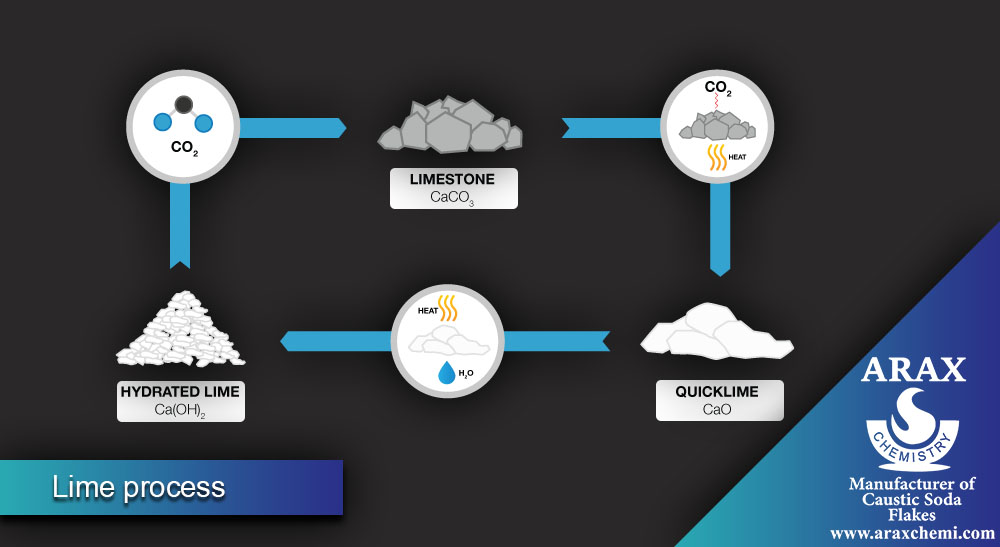
In the lime process, soda ash is also produced, but using different raw materials. The main steps of this process are:
- Cooling and purifying the placebo: The prepared placebo (sodium chloride in water) is cooled and purified from impurities.
- Addition of calcium carbonate: Calcium carbonate is added to the placebo, which forms the semi-product of Sodium carbonate as a calcium carbonate precipitate.
- Separation of soda ash: The precipitate formed is separated from the solution and then converted into pure soda ash using methods such as purification and drying.
Innovations of soda ash
Recent efforts have been made to optimize Sodium carbonate production processes using greener and more sustainable methods and technologies. This research has been carried out in order to reduce energy consumption, reduce environmental effects and improve resource efficiency. Researches in the field of improving soda ash purification methods in order to remove pollution and impurities have been investigated. This research includes the use of advanced filtration, absorption and extraction processes of contaminated materials and the use of new catalysts. New uses of soda ash: Recent research has been done in the field of new uses of soda ash. For example, the use of sodium carbonate in the production of recyclable and reusable batteries, use in catalytic processes, use in nano technologies, etc. are among the things that have been investigated.
Improving the quality and properties of soda ash: Research has been conducted in the field of improving the quality and properties of soda ash. Such as improving purity and degradability, optimizing physical and chemical properties, improving resistance to moisture and heat, etc.
It is important to know that these researches and innovations are based on the fields studied and developed, and more detailed information about them may be available in scientific sources and related articles.
last word
Soda ash, or soda ash, is an important chemical used in many industries, including glass production, soap, detergents, paper, food, and petrochemicals. In the processes of refining vegetable oils, sodium carbonate plays a crucial role as a substance used for oil separation, acid neutralization, pH adjustment, and purification. In desulfurization processes, sodium carbonate is utilized as a key material to separate sulfur from fossil fuels, such as crude oil and natural gas, thereby improving fuel quality. Nowadays, the lime method is more commonly used due to its higher efficiency and lower energy consumption. As a versatile and essential chemical, sodium carbonate has a significant impact on various industries.
Frequently asked questions about sodium carbonate
1. What is sodium carbonate, and what are its applications?
Sodium carbonate is an alkaline substance, also known as soda ash or soda salt. It is used in many industries, such as:
- Glass production: Helps lower the melting point of glass.
- Soap and detergent production: Acts as a booster in cleaning agents.
- Food industry: Used as a pH regulator or preservative.
- Water treatment: Utilized for softening water and removing magnesium and calcium ions.
2. Is soda ash dangerous?
Sodium carbonate is generally considered a relatively safe substance and is used in many household and industrial products. However, direct contact can cause eye and skin irritation, and consuming large amounts may damage the digestive system.
3. How can soda ash be used at home?
- Surface cleaning: As a natural cleaner for degreasing and cleaning various surfaces.
- Water softening: Helps soften water when washing clothes.
- Baking: Used as an alkalizing agent in making certain types of bread and pastries.
4. Does sodium carbonate react with other chemicals?
Yes, sodium carbonate reacts with acids to produce carbon dioxide (CO2) gas. This reaction is particularly used in chemical processes to produce CO2.
5. What is the difference between sodium carbonate and sodium bicarbonate?
Sodium carbonate (Na2CO3) and sodium bicarbonate (NaHCO3) are different compounds. Sodium bicarbonate, commonly known as baking soda, is mainly used for cooking and medicinal purposes, while sodium carbonate is used more in chemical industries and glass production.
6. Does sodium carbonate naturally occur in nature?
Yes, sodium carbonate occurs naturally in a mineral called trona, which is the primary source for its industrial production.


In detergent making, does Soda Ash get dissolved in water first before being mixed with other chemicals?
Yes, in the manufacture of detergents, soda ash (sodium carbonate) is usually dissolved in water before being mixed with other chemicals. This is done to ensure that the soda ash is completely dissolved and evenly distributed throughout the mixture, which helps improve the overall quality and consistency of the detergent. Once dissolved, it can be combined with other ingredients such as surfactants, builders, and enzymes. The solubility of soda ash in water also allows it to react with other ingredients, enhancing its role as a water softener and pH adjuster in the final product.
Greatest news for all us
Awesome news for all us
[u]Unlish your wild nature with hot girls[/u] – [url=https://bit.ly/3ZZbjN2][b]click here![/b][/url]
[url=https://bit.ly/3C6dEOb]XrumerAI+Xevil+Hrefer+SocPlugin – an invincible combination for successful monetization![/url]
Chaming news for all us
[b]Expose your savageness[/b] – [url=https://psee.io/663bfu][b]appease your sexual passion right now![/b][/url]
[b]Reveal your savageness[/b] – [url=https://psee.io/663bg2][b]meet your sexual fervor right now![/b][/url]
[b][url=https://bit.ly/3C6dEOb]XrumerAI+Xevil+Hrefer+SocPlugin – an invincible combination for successful monetization![/url][/b]
[b]Demonstrate your wildness[/b] – [url=https://psee.io/663bfd][b]satisfy your sexual passion right now![/b][/url]
[b][url=https://bit.ly/3C6dEOb]XrumerAI+Xevil+Hrefer+SocPlugin – an invincible combination for successful monetization![/url][/b]
Finest news for all us
[url=https://bit.ly/3EGKSVc][img]https://tripacostarica.com/1/tp/sr/1.png[/img][/url]
[b][u]See[/u][/b] yourself settled on the deck of a catamaran, a fresh breeze fumbling with your hair, and around you spreading an boundless horizon, where the water mixes with the air. There is no rush of cities, no buzz of cars, just you and world in its natural beauty. Hiring a motorboat is not just a journey, it is an ability to sense unlimited autonomy of action and a profound connection with the external world, which is difficult to find in routine life. Why not undertake this step right now?
Freedom is what is so lacking in the recent individual, caught in plans, tasks and perpetual notifications. When you rent a ship, you become the master of your path. Want to head to a secluded beach where no one interrupts your seclusion? Please! Want to discover undiscovered bays and islands that only native fishermen know about? Easy! No established routes, no restrictions — just you and your wishes. You can rise at dawn to see the sun coloring the water in golden hues, or spend the day plunging among marine reefs abundant in life. Booking a motorboat offers you ability, allowing you to feel as free as the wind that fills the sails.
[url=https://bit.ly/3EGKSVc][img]https://tripacostarica.com/1/tp/sr/2.png[/img][/url]
[b][u]But[/u][/b] self-determination is only one side of the medal. The other is a strong link with the outdoors that cannot be realized while sitting in an cubicle or walking on sidewalk. Waves is a living organism that sings, shares secrets and sometimes tests. Catching the sound of the sea, noticing the salty air and watching the ballet of the dolphins, you begin to understand how trivial our routine bustle is compared to the magnitude of nature. Yacht becomes your passage into this world: you can cast anchor in the middle of the water, to gaze at the stars that seem more vivid than ever, or host a picnic on the deck, enveloped only by the echoes of nature.
Chartering a vessel suits not only lone travelers seeking inner peace, but also for those who esteem connection. See this: you with companions or kin venturing on a journey, recounting stories under the celestial bodies, playing tabletop games on the deck, or simply savoring the silence together. Boat becomes your exclusive shelter, where there is no room for dull routines or alien distractions. You can throw a romantic dinner at sunset, watching as the sky paints a palette of reddish and deep shades, or host a exciting party with melodies and dancing under the open sky. Every event on the boat is a window to create memories that will comfort you for many years.
[u][b]Of course[/b][/u], chartering a ship is also an way to relish ease. Modern motorboats are outfitted with all the required: from ample cabins and kitchens to spas and entertainment systems. You can feel like you’re at a top-tier resort, but with the edge of utter mobility. Want to moor in a place where there isn’t a single accommodation? No problem! A motorboat will deliver you where no plane or aircraft can reach. This notion of exclusivity and autonomy makes renting a ship a unique experience that cannot be compared with anything else.
[url=https://bit.ly/3EGKSVc][img]https://tripacostarica.com/1/tp/sr/3.png[/img][/url]
But the [b][u]most crucial[/u][/b] thing is that booking a motorboat is accessible to anyone who aspires of liberty and excursions. You don’t have to be an trained sailor — qualified crews and straightforward vessels will make your trip protected and enjoyable. You can choose a vessel of any dimension and class: from compact models for couples to opulent vessels for large gatherings. The method of renting is uncomplicated: just define your needs, decide on a route, and confide in the professionals. In just a few days, you will be able to be on the deck, noticing your heart beat in unison with the waves.
Don’t delay your visions for later. Full autonomy of action and deep harmony with the world are waiting for you. Hire a motorboat right now and find a world where there are no boundaries except those you establish yourself. The sea urges — respond its whisper, and you will understand what actual freedom is. It’s not just a voyage, it’s a approach that drives, revives, and enriches with meaning. Do the first step — and let the wind lead you to where wishes become truth!
Complete freedom of action and deep unity with nature!
[url=https://bit.ly/3EGKSVc][b]Try it right now )[/b][/url]
Great news for all us
[url=https://bit.ly/3EGKSVc][img]https://tripacostarica.com/1/tp/sr/1.png[/img][/url]
[b][u]Think[/u][/b] yourself settled on the deck of a vessel, a cool breeze fumbling with your hair, and around you stretching an boundless horizon, where the depths flows with the heights. There is no chaos of cities, no buzz of cars, just you and world in its pure beauty. Chartering a ship is not just a journey, it is an opportunity to grasp unlimited independence of action and a strong harmony with the external world, which is difficult to find in normal life. Why not perform this step now?
Autonomy is what is so lacking in the modern man, stuck in timetables, obligations and continuous notifications. When you charter a yacht, you become the commander of your course. Want to travel to a secluded beach where no one disturbs your peace? Please! Want to explore hidden bays and islands that only resident fishermen know about? Easy! No pre-set routes, no restrictions — just you and your aspirations. You can start at dawn to see the sun coloring the water in yellow hues, or employ the day swimming among underwater reefs abundant in life. Chartering a yacht provides you wings, allowing you to be as liberated as the wind that powers the sails.
[url=https://bit.ly/3EGKSVc][img]https://tripacostarica.com/1/tp/sr/2.png[/img][/url]
[b][u]But[/u][/b] freedom is only one side of the chip. The other is a intense connection with the outdoors that cannot be realized while sitting in an desk or walking on surface. Waves is a pulsing organism that breathes, hints and sometimes challenges. Catching the sound of the ocean swells, feeling the salty air and admiring the dance of the dolphins, you begin to understand how small our routine bustle is compared to the splendor of nature. Vessel becomes your gateway into this world: you can lower anchor in the middle of the water, to contemplate the stars that seem more vivid than ever, or host a picnic on the deck, immersed only by the rustles of nature.
Booking a motorboat suits not only solitary travelers looking for inner peace, but also for those who cherish socializing. Picture this: you with loved ones or kin sailing on a excursion, discussing stories under the celestial bodies, playing deck games on the deck, or simply relishing the silence together. Boat becomes your personal haven, where there is no room for dreary routines or alien distractions. You can arrange a passionate dinner at sunset, observing as the sky paints a palette of warm and deep shades, or put on a lively party with music and sways under the endless sky. Every instance on the yacht is a opportunity to create recollections that will reassure you for endless years.
[u][b]Of course[/b][/u], chartering a boat is also an chance to relish ease. Contemporary yachts are fitted with all the vital: from roomy cabins and kitchens to spas and diversion systems. You can think like you’re at a luxury resort, but with the plus of full mobility. Want to pause in a place where there isn’t a single lodge? No problem! A boat will bring you where no bus or airplane can reach. This feeling of exclusivity and self-sufficiency makes booking a boat a distinctive experience that cannot be equated with anything else.
[url=https://bit.ly/3EGKSVc][img]https://tripacostarica.com/1/tp/sr/3.png[/img][/url]
But the [b][u]most significant[/u][/b] thing is that chartering a motorboat is open to anyone who longs of self-determination and journeys. You don’t have to be an trained sailor — qualified crews and simple vessels will make your adventure protected and delightful. You can choose a vessel of any capacity and category: from little models for partners to lavish vessels for large crowds. The process of renting is uncomplicated: just define your preferences, identify a route, and entrust to the professionals. In just a few days, you will be able to be on the deck, perceiving your heart beat in rhythm with the currents.
Don’t defer your visions for tomorrow. Utter independence of action and deep unity with the world are waiting for you. Book a motorboat right now and explore a world where there are no boundaries except those you establish yourself. Ocean beckons — respond its voice, and you will see what genuine autonomy is. It’s not just a adventure, it’s a mode that drives, refreshes, and suffuses with purpose. Take the first step — and let the wind lead you to where visions become fact!
Complete freedom of action and deep unity with nature!
[url=https://bit.ly/3EGKSVc][b]Try it right now )[/b][/url]
[url=https://bit.ly/3EGKSVc][img]https://tripacostarica.com/1/tp/sr/1.png[/img][/url]
[b][u]Picture[/u][/b] yourself standing on the deck of a yacht, a gentle breeze fluttering with your hair, and around you spreading an limitless horizon, where the water merges with the heights. There is no rush of cities, no sound of cars, just you and world in its pure beauty. Renting a motorboat is not just a trip, it is an chance to feel full independence of action and a intense unity with the natural world, which is difficult to discover in ordinary life. Why not do this step without delay?
Autonomy is what is so lacking in the recent man, trapped in plans, tasks and infinite notifications. When you charter a boat, you become the captain of your fate. Want to travel to a hidden beach where no one invades your solitude? Please! Want to explore mysterious bays and islands that only local fishermen know about? Easy! No pre-set routes, no constraints — just you and your longings. You can start at dawn to see the sun illuminating the water in warm hues, or use the day plunging among underwater reefs abundant in life. Renting a boat grants you freedom, allowing you to be as unrestricted as the wind that swells the sails.
[url=https://bit.ly/3EGKSVc][img]https://tripacostarica.com/1/tp/sr/2.png[/img][/url]
[b][u]But[/u][/b] liberty is only one side of the token. The other is a strong affinity with the world that cannot be perceived while sitting in an room or roaming on sidewalk. Waves is a pulsing organism that murmurs, murmurs and sometimes tests. Attending to the sound of the waves, noticing the salty air and noticing the play of the dolphins, you begin to understand how trivial our daily bustle is compared to the majesty of nature. Cruiser becomes your passage into this world: you can lower anchor in the middle of the sea, to contemplate the stars that seem more proximate than ever, or plan a picnic on the deck, surrounded only by the sounds of nature.
Renting a boat suits not only single travelers seeking spiritual peace, but also for those who appreciate socializing. Picture this: you with mates or loved ones setting out on a journey, sharing stories under the stars, playing tabletop games on the deck, or simply delighting in the silence together. Yacht becomes your individual haven, where there is no room for monotonous routines or alien distractions. You can set up a tender dinner at sunset, observing as the sky paints a array of orange and violet shades, or host a energetic party with rhythms and grooves under the starlit sky. Every instance on the ship is a window to create memories that will reassure you for many years.
[u][b]Of course[/b][/u], using a ship is also an chance to enjoy convenience. Current yachts are supplied with all the essential: from ample cabins and kitchens to hot tubs and entertainment systems. You can feel like you’re at a five-star resort, but with the advantage of full mobility. Want to stay in a place where there isn’t a single inn? No problem! A boat will carry you where no car or airplane can reach. This feeling of prestige and independence makes chartering a boat a remarkable experience that cannot be equated with anything else.
[url=https://bit.ly/3EGKSVc][img]https://tripacostarica.com/1/tp/sr/3.png[/img][/url]
But the [b][u]most crucial[/u][/b] thing is that using a boat is offered to anyone who dreams of freedom and journeys. You don’t have to be an knowledgeable sailor — qualified crews and manageable vessels will make your trip protected and agreeable. You can select a boat of any dimension and category: from little models for couples to opulent vessels for large crowds. The step of renting is easy: just set your needs, select a route, and entrust to the professionals. In just a few days, you will be able to stand on the deck, feeling your heart beat in unison with the rolls.
Don’t defer your wishes for later. Utter self-determination of action and genuine harmony with the environment are waiting for you. Charter a vessel immediately and discover a world where there are no boundaries except those you establish yourself. Water urges — reply its summons, and you will understand what real freedom is. It’s not just a expedition, it’s a lifestyle that inspires, revives, and imbues with value. Make the first step — and let the wind transport you to where aspirations become actuality!
Complete freedom of action and deep unity with nature!
[url=https://bit.ly/3EGKSVc][b]Try it right now )[/b][/url]
[url=https://bit.ly/3EGKSVc][img]https://tripacostarica.com/1/tp/sr/1.png[/img][/url]
[b][u]Think[/u][/b] yourself located on the deck of a yacht, a soft breeze fluttering with your hair, and around you stretching an enormous horizon, where the water connects with the heights. There is no chaos of cities, no thunder of cars, just you and landscape in its wild beauty. Renting a ship is not just a journey, it is an ability to perceive full liberty of action and a strong union with the surrounding world, which is difficult to obtain in daily life. Why not make this step immediately?
Autonomy is what is so lacking in the current man, trapped in schedules, commitments and perpetual notifications. When you hire a ship, you become the leader of your destiny. Want to journey to a secluded beach where no one disrupts your quiet? Please! Want to venture into hidden bays and islands that only native fishermen know about? Easy! No predetermined routes, no obstacles — just you and your desires. You can arise at dawn to see the sun flooding the water in golden hues, or pass the day diving among coral reefs abundant in life. Hiring a motorboat grants you ability, allowing you to feel as free as the wind that powers the sails.
[url=https://bit.ly/3EGKSVc][img]https://tripacostarica.com/1/tp/sr/2.png[/img][/url]
[b][u]But[/u][/b] freedom is only one side of the marker. The other is a deep link with the outdoors that cannot be felt while sitting in an room or walking on asphalt. Waves is a living organism that breathes, whispers and sometimes tests. Catching the sound of the surf, sensing the salty air and admiring the sway of the dolphins, you begin to see how petty our ordinary bustle is compared to the majesty of nature. Cruiser becomes your passage into this world: you can throw anchor in the middle of the water, to reflect on the stars that seem more vivid than ever, or host a picnic on the deck, surrounded only by the sounds of nature.
Chartering a ship suits not only individual travelers aiming for spiritual peace, but also for those who value interaction. See this: you with companions or household heading out on a sail, telling stories under the heavens, playing game games on the deck, or simply enjoying the silence together. Vessel becomes your private haven, where there is no room for dull routines or unwanted distractions. You can throw a loving dinner at sunset, noticing as the sky becomes a palette of reddish and deep shades, or host a energetic party with rhythms and dancing under the open sky. Every occasion on the vessel is a chance to create remembrances that will warm you for many years.
[u][b]Of course[/b][/u], using a motorboat is also an way to relish luxury. Advanced motorboats are outfitted with all the needed: from ample cabins and prep spaces to hot tubs and leisure systems. You can think like you’re at a luxury resort, but with the benefit of total mobility. Want to pause in a place where there isn’t a single resort? No problem! A boat will take you where no plane or aircraft can reach. This sense of uniqueness and autonomy makes renting a boat a singular experience that cannot be paralleled with anything else.
[url=https://bit.ly/3EGKSVc][img]https://tripacostarica.com/1/tp/sr/3.png[/img][/url]
But the [b][u]most significant[/u][/b] thing is that hiring a motorboat is available to anyone who hopes of freedom and explorations. You don’t have to be an seasoned sailor — qualified crews and simple vessels will make your journey secure and satisfying. You can select a ship of any scale and grade: from compact models for couples to grand vessels for large groups. The process of renting is simple: just define your needs, decide on a route, and trust the professionals. In just a few days, you will be able to sit on the deck, experiencing your heart beat in harmony with the currents.
Don’t defer your hopes for afterwards. Complete autonomy of action and deep alliance with the outdoors are waiting for you. Lease a ship immediately and explore a world where there are no restrictions except those you impose yourself. Waves invites — answer its cry, and you will realize what true self-determination is. It’s not just a expedition, it’s a lifestyle that stimulates, refreshes, and permeates with significance. Take the first step — and let the wind carry you to where aspirations become truth!
Complete freedom of action and deep unity with nature!
[url=https://bit.ly/3EGKSVc][b]Try it right now )[/b][/url]
[b]Desire to revive instantly[/b] – [u]pick a stunning lady, dude or transgender![/u]
[b]There is a large selection of enticing shapes for you[/b] – [u]fresh, beautiful, sexy![/u]
[b][url=https://psee.io/663bfg]Fuck their instantly![/url][/b]
[b]Need to energize fast[/b] – [u]select a attractive chick, dude or shemale![/u]
[b]There is a huge range of juicy physiques for you[/b] – [u]fresh, stunning, irresistible![/u]
[b][url=https://psee.io/663bfz]Hump their instantly![/url][/b]
[b]Crave to boost swiftly[/b] – [u]pick a sexy babe, man or shemale![/u]
[b]There is a wide variety of luscious forms for you[/b] – [u]youthful, beautiful, sexy![/u]
[b][url=https://psee.io/663bfq]Bang their instantly![/url][/b]
[b]Want to energize quickly[/b] – [u]take a sexy girl, guy or transgender![/u]
[b]There is a big choice of enticing forms for you[/b] – [u]new, beautiful, sexy![/u]
[b][url=https://psee.io/663bfe]Shag their immediately![/url][/b]
[b]Want to revive swiftly[/b] – [u]choose a sexy girl, gent or trans![/u]
[b]There is a big range of delicious bodies for you[/b] – [u]teen, stunning, irresistible![/u]
[b][url=https://psee.io/663bfr]Fuck their now![/url][/b]
[b]Your full authority[/b] over powerful functional equipment – [u]you transform into a racer, an aviator and a pioneer![/u]
[b]Modify and improve[/b] unit just for you – [u]study, research, explore your surroundings in your own way![/u]
[b]Experience unforgettable pleasure[/b] and amazing emotions – [u]thanks to your victories plus efforts![/u]
[url=https://psee.io/7dcler][b]Click here to purchase[/b][/url] cool unit which your friend doesn’t have [u]right now![/u]
[url=https://psee.io/7fxb3x][img]https://tripacostarica.com/1/tr/1.png[/img][/url]
[b]Summer this year[/b] presents fantastic opportunities for budget-conscious travelers looking for unforgettable experiences without emptying the bank.
To optimize value, think about destinations and strategies that harmonize affordability with adventure.
Eastern Europe, like Poland or Hungary, is a gem—vibrant cities like Krakow or Budapest present rich history, stunning architecture, and delicious cuisine at a slice of Western Europe’s costs.
[url=https://psee.io/7fxb3x][img]https://tripacostarica.com/1/tr/2.png[/img][/url]
[b]Hostels and Airbnb[/b] rentals launch at $20–$30 per night, and hearty meals cost under $10. Southeast Asia, including Vietnam and Thailand, stays a top pick for tropical vibes.
Consider Hanoi’s bustling markets or Chiang Mai’s serene temples, with street food at $1–$3 and guesthouses around $15.
[url=https://psee.io/7fxb3x][img]https://tripacostarica.com/1/tr/3.png[/img][/url]
[b]For North Americans[/b], Mexico’s Riviera Maya fuses pristine beaches with cultural sites like Tulum, where all-inclusive deals begin at $80/night.
Book flights early, use fare alerts, and opt for public transport to save. Traveling off-peak (June or late August) lowers costs further.
With wise planning, your summer escape can be both affordable and incredible!
[url=https://psee.io/7fxb3x][b][u]Get your value-for-money travel right now![/u][/b][/url]
[b]Your total command[/b] over dynamic functional gadget – [u]you transform into a competitor, a navigator along with a discoverer![/u]
[b]Customize and upgrade[/b] model to your liking – [u]study, investigate, discover the world as you want![/u]
[b]Experience memorable delight[/b] and indescribable feelings – [u]through your accomplishments as well as outcomes![/u]
[url=https://psee.io/7dcler][b]Click here to buy[/b][/url] cool unit what your friend doesn’t have [u]right now![/u]
[b]Your absolute command[/b] of robust practical gadget – [u]you are a driver, an aviator as well as a discoverer![/u]
[b]Modify and boost[/b] system to suit you – [u]study, investigate, discover the world in your own way![/u]
[b]Get lasting delight[/b] and amazing sensations – [u]through your victories as well as results![/u]
[url=https://psee.io/7dcler][b]Click here to purchase[/b][/url] fantastic gadget that your buddy is missing [u]right now![/u]
[url=https://psee.io/7fxb3x][img]https://tripacostarica.com/1/tr/1.png[/img][/url]
[b]Summer this year[/b] offers thrilling opportunities for budget-conscious travelers looking for unforgettable experiences without breaking the bank.
To optimize value, check out destinations and strategies that combine affordability with adventure.
Eastern Europe, like Poland or Hungary, is a gem—vibrant cities like Krakow or Budapest feature rich history, stunning architecture, and delicious cuisine at a fraction of Western Europe’s costs.
[url=https://psee.io/7fxb3x][img]https://tripacostarica.com/1/tr/2.png[/img][/url]
[b]Hostels and Airbnb[/b] rentals open at $20–$30 per night, and hearty meals cost under $10. Southeast Asia, including Vietnam and Thailand, persists a top pick for tropical vibes.
Think Hanoi’s bustling markets or Chiang Mai’s serene temples, with street food at $1–$3 and guesthouses around $15.
[url=https://psee.io/7fxb3x][img]https://tripacostarica.com/1/tr/3.png[/img][/url]
[b]For North Americans[/b], Mexico’s Riviera Maya fuses pristine beaches with cultural sites like Tulum, where all-inclusive deals open at $80/night.
Reserve flights early, use fare alerts, and pick for public transport to save. Traveling off-peak (June or late August) trims costs further.
With clever planning, your summer getaway can be both affordable and awesome!
[url=https://psee.io/7fxb3x][b][u]Get your value-for-money travel right now![/u][/b][/url]
[b]Name novel[/b] pills to massive orgasms – more also more vivid peaks!
[b]by seen[/b] previously – greatly recent inside healing study study!
[b]100% secure[/b] as effective – as enhanced passion also still elevated penile firmness!
[url=https://psee.io/7qlgd7][b]Enhance capacity from own emissions promptly right now![/b][/url]
[u][b]Us hurrying towards your help[/b][/u]—one group with experts whom shall fast repair a aftermath of some drainage urgency inside their home!
[u][b]Your quick response[/b][/u] – the core towards decreasing thy charges by certain subsequent large mends!
[url=https://5.gp/b31q6i3y][b]Experience our speed and excellence with support right now![/b][/url]
[u][b]We’re all hurrying into their help[/b][/u]—one unit by masters that would quickly mend this consequences from one hydraulics urgency on thy house!
[u][b]Your quick reply[/b][/u] – some core for minimizing thy charges of all ensuing major restorations!
[url=https://5.gp/b31q6i3y][b]Test my speed together merit among service right now![/b][/url]
[u][b]We are hastening to their aid[/b][/u]—a team of specialists whose would quickly restore the results from the hydraulics emergency inside your dwelling!
[u][b]One’s fast reaction[/b][/u] – the key for cutting our outlays for some subsequent significant corrections!
[url=https://5.gp/b31q6i3y][b]Experience their quickness plus standard from support right now![/b][/url]
[b]Brand new[/b] supplements to enormous ecstasies – more well additional fierce climaxes!
[b]ever viewed[/b] prior – extremely latest within therapeutic research study!
[b]Totally harmless[/b] as potent – well increased drive and still elevated sexual firmness!
[url=https://psee.io/7qlgd7][b]Elevate quantity in personal expulsions now right now![/b][/url]
[u][b]We hurrying to their relief[/b][/u]—one squad by specialists who would swiftly fix this results among the piping disaster in your residence!
[u][b]My quick reply[/b][/u] – a solution towards cutting one’s charges in every following large restorations!
[url=https://5.gp/b31q6i3y][b]Test their rapidity together excellence from help right now![/b][/url]